Research

Research in the Emslie group is focused in 3 areas:
(1) Electropositive Metal Complexes of Extremely Rigid Pincer Ligands: Organometallic Reactivity and Alkane Complexes.
(2) Group 13 Lewis Acid Appended Transition Metal Complexes: Metal-Ligand Bonding and Cooperative Catalysis.
(3) New Organometallic Precursors and Reactivity for Metal ALD (Atomic Layer Deposition).
(4) Organomanganese Chemistry.
Each of the above topics combines fundamental/exploratory organometallic chemistry with at least one target application that has not yet been realized, and is of high current importance. These 4 research areas are described in more detail below:
(1) Electropositive Metal Complexes of Extremely Rigid Pincer Ligands
All pincer ligands employed in this research are of our own design. These ligands are based on a highly rigid tricyclic backbone (e.g. a xanthene, thioxanthene or acridanide backbone) in order to provide access to coordination environments which are dictated by design rather than the preferences of the central metal, to ensure that steric bulk is positioned so as to maximize its effectiveness, and to create a rigid hydrophobic pocket around the metal center. Sample ligands are shown here:
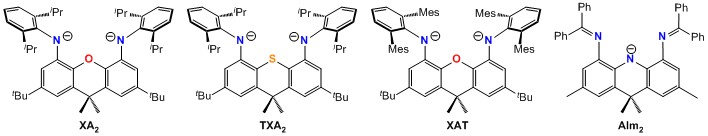
Our rigid pincer ligand research is divided into three themes (a-c below):
(a) Thermally Robust Rare Earth and Actinide Complexes for Small Molecule Activation and Catalysis
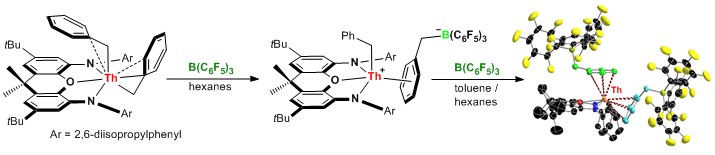
Our current research involves thorium, uranium and/or yttrium, and is focused on the synthesis of thermally robust neutral and cationic alkyl, allyl and hydride complexes. Rigid pincer ligand dianions are most suitable for development of the chemistry of tetravalent actinide metals, while monoanions are better suited for the synthesis of trivalent rare earth complexes with two reactive valences. New alkyl and hydride compelxes are investigated for applications in olefin polymerization and hydroamination, with a focus the development of catalysts with stability at high temperature. An example of cation and dication synthesis by single and double alkyl anion abstraction from thorium is shown below.
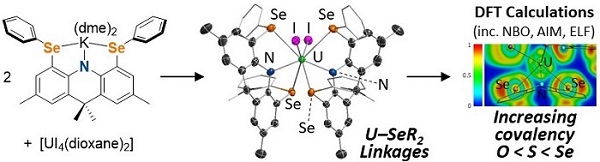
(b) Rigid ligands to promote the formation of actinide-soft donor linkages
Rigid ligands have also been used to promote the formation of unique actinide-soft donor linkages of potential relevance to lanthanide-actinide separation in nuclear fuel reprocessing. For example, in 2022 we reported the first uranium-selenoether linkages utilizing a rigid monoanionic SeNSe-donor ligand.
(c) Metal-Alkane Complexes
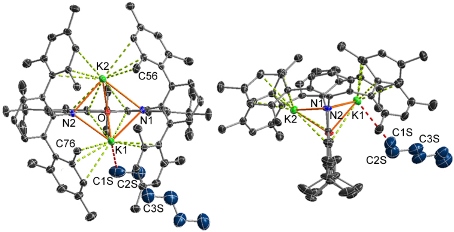
The dipotassium salt of the highly rigid XAT pincer ligand (shown above) was crystallized as from hexane/toleune, and an X-ray structure of the resulting crystals of K2[XAT]·hexane revealed close approach of hexane to potassium. Similar solid state K–H3CR or K–H2CR2 interactions were observed when K2[XAT] was crystallized from n‑pentane, 3-methylpentane, cyclopentane, toluene and O(SiMe3)2. The 3-methylpentane structure was investigated computationally, revealing a K–alkane interaction that is essentially ionic, and supported by van der Waals interactions between the hydrocarbon and the hydrophobic binding pocket.
This work provided the first examples of main group metal–alkane interactions, and is only the fourth report of metal–alkane interactions observed in the solid state (the other examples are an iron complex reported by Reed, uranium complexes reported by Mayer, and a rhodium complex reported by Weller).
Our current research is focused on further developing the chemistry of the XAT ligand, and related pincer ligands, for the systematic synthesis of metal–alkane complexes differing in the identity of the central metal. Interactions of this type are of great interest given their involvement en-route to alkane C–H bond activation; an economically and environmentally attractive prospect, when coupled with functionalization.
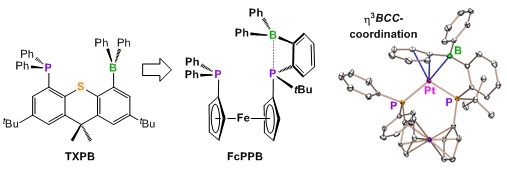
(2) Group 13 Lewis Acid Appended Transition Metal Complexes: Studies of Metal-Ligand Bonding and Cooperative Catalysis.
In our original work in this field, we reported a rare example of an isolable ambiphilic ligand, TXPB (shown below), which contains a phosphine, a thioether, and a triarylborane. This ligand yielded the first examples of η3BCC-arylborane complexes, and also allowed exploration of a broad range of metal–X–borane (X = F, Cl, Br and I), metal–enone–borane and metal–isonitrile–borane interactions in complexes of Rh, Ni, Pd and Pt. These studies provided a detailed picture of the behaviour of pendent arylborane Lewis acids in combination with a variety of metals and co-ligands. However, the Achilles heel of the TXPB ligand is the central thioether donor, which readily dissociates from the metal centre. To address this limitation, a new much more electron-donating borane-containing ambiphilic ligand, FcPPB (shown below), was prepared.
FcPPB and TXPB are the only tridentate ambiphilic ligands in which a borane is positioned at the extremity of the ligand framework; this is desirable as a means to encourage the pendent borane to interact with substrates and co-ligands, with a view towards accessing cooperative reactivity (reactivity which relies upon both the metal and the borane). Furthermore, the FcPPB ligand is a borane-substituted analogue of dppf [1,1'-bis(diphenylphosphino)ferrocene]; a wide bite-angle bis-phosphine ligand used extensively in late TM catalysis.
Our current research is focused on exploring the reactivity of FcPPB, and related ligands bearing different Lewis acidic groups. We are particularly interested in studies of metal-Lewis acid bonding, and the development of cooperative catalytic reactivity involving alkenes and CO.
(3) New Organometallic Precursors and Reactivity for Metal ALD (Atomic Layer Deposition).
Background: ALD is a technique for deposition of ultra-thin films with a range of technological applications. The attraction of ALD is that the resulting films are more uniform and conformal than those deposited using other methods such as CVD (chemical vapor deposition) or PVD (physical vapour deposition). Applications of ALD include the deposition of HfO2 in computer chips, Al2O3 in Dynamic Random Access Memory, and ZnS in electroluminescent displays. ALD has also found emerging applications in the development of memory devices, solar cells, optical communications components, sensors, and batteries.
Film deposition in ALD is achieved using repeated surface-based reactions between a volatile metal precursor and a volatile co-reactant. The precursor and co-reactant are delivered to the substrate surface in the vapour phase (with precursor and co-reactant pulses separated by inert gas purge steps to avoid gas-phase reactivity), and the chemistry is such that the thickness of the resulting film depends only on the number of deposition cycles, and not on the amount of precursor and co-reactant delivered (so long as the amount is above a certain minimum value); this is termed self-limiting behaviour. Thermal ALD utilizes reactions with stable co-reactant molecules, whereas plasma-enhanced ALD utilizes plasma-generated radicals; from herein ALD is used to refer to thermal ALD.
Typical ALD co-reactants are H2O, O2 or O3 for metal oxide ALD, NH3 for metal nitride ALD, and H2S for metal sulfide ALD. ALD of pure elements is arguably more challenging, given that it typically involves reduction of an element (in a precursor molecule) in a positive oxidation state to the zero oxidation state, using only volatile reagents and producing only volatile byproducts. Consequently, a range of strategies have had to be employed, depending on the metal and the exact nature of the precursor complex.
Our Research: Our initial publications in the field focused on copper metal, but a range of metals, including more electropositive metals such as manganese, are now under investigation (some potential mixed alkyl/allyl precursor molecules are shown below). Electropositive metal ALD is particularly challenging given the reluctance of electropositive metals to be reduced from a positive oxidation state to the zero oxidation state, and the requirement for both the reducting agent and the byproducts to be volatile. Recent work has also involved main group element ALD, including the first room temperature ALD process for pure element (Sb) deposition. Collaborative projects focused on ALD of various other materials are also underway. Our research typically progresses from the design, synthesis and characterization of new precursor molecules (which must be volatile, thermally robust, and highly reactive towards the envisaged co-reactants), to solution reactivity studies, and then ALD reactor studies and characterization of the resulting thin films.
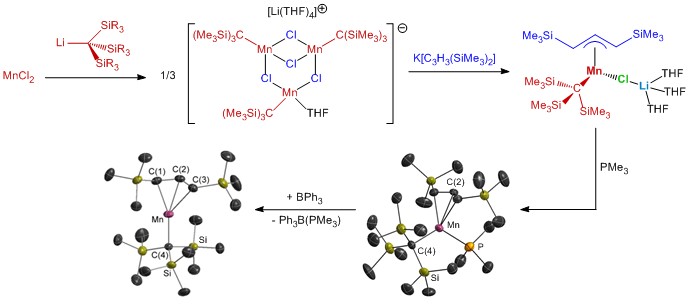
(4) Organomanganese chemistry.
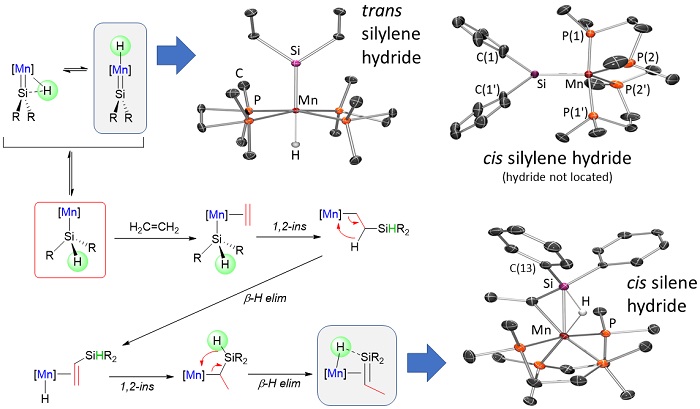
Our research in ALD of manganese-containing films led to the synthesis of a range of manganese(II) alkyl and allyl complexes. Additionally, manganese(I) complexes were explored as potential ALD precursors, and during the course of this work, unique reactivity leading to (a) manganese silylene hydride, and (b) silene hydride complexes was discovered; the silene hydride complexes are the only examples of sterically unstabilized silene complexes of a first row transition metal. Reactivity stemming from these complexes (and related disilyl hydride and silyl dihydride complexes) is currently under investigation and is of interest from a fundamental perspective, and potentially for applications in catalysis.