Note: this page is under construction - some of the images are low resolution, and will be replaced with higher resolution images over the next few months.
Contents:
- Alane-appended platinum complexes
- FcPPB platinum complexes
- Rh TXPB complexes
- Rh-Fe heterobimetallic complexes of TXPB
- Pd complexes of TXPB
- Pt alkyl and aryl complexes of TXPB
- PS- and PSP-donor ligand complexes
- Vinylborane complexes of Pt and Ni
- Th complexes
- U complexes
- Y complexes
- Alkali metal complexes
- Mn complexes
- Cu, Zn and Al complexes formed en route to copper metal deposition
An Alane-Appended Analogue of DPPF and its Platinum Complexes
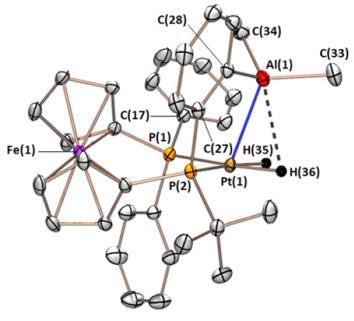
We recently prepared an alane-appended analogue of dppf (dppf is a wide bite-angle bis-phosphine ligand used extensively in late transition metal catalysis). The ligand, FcPPAl:
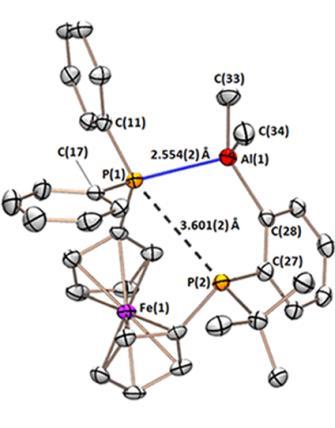
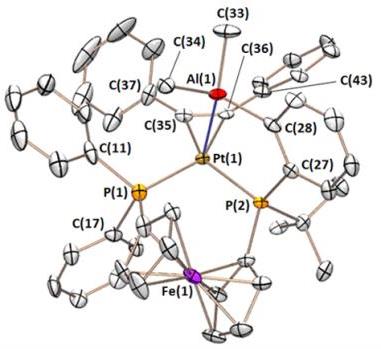
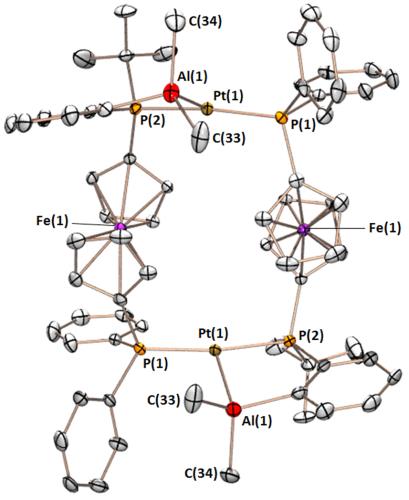
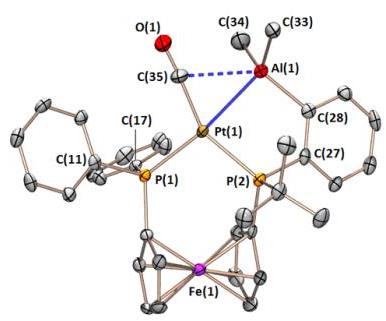
The FcPPAl ligand binds tenaciously to platinum in a range of oxidation states, yielding complexes with square pyramidal, T-shaped, and distorted square planar geometry. The CO and dihydride complexes also feature unusual multiceter bonding between the alane, platinum, and either a carbonyl or a hydride ligand.
|
A Borane-Appended Analogue of DPPF and its Platinum Complexes
We recently prepared a borane-appended analogue of dppf (dppf is a wide bite-angle bis-phosphine ligand used extensively in late transition metal catalysis). The ligand, FcPPB:
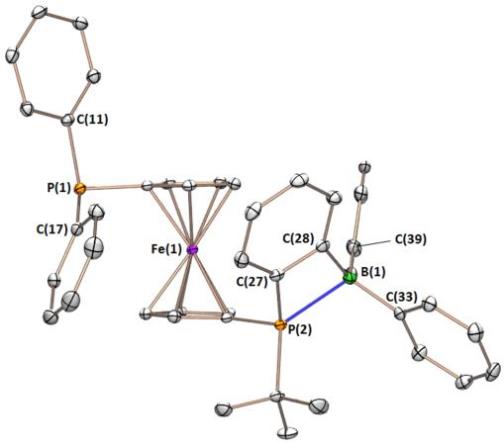
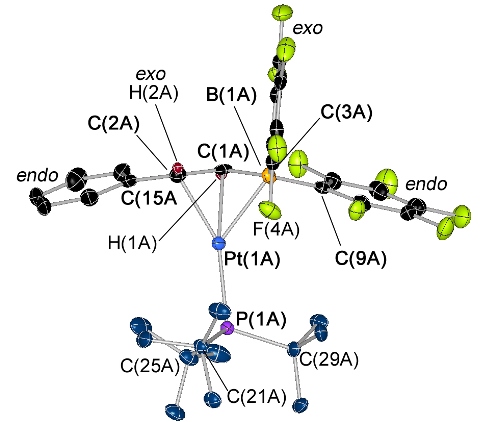
The FcPPB ligand η3BCC-coordinated to platinum:
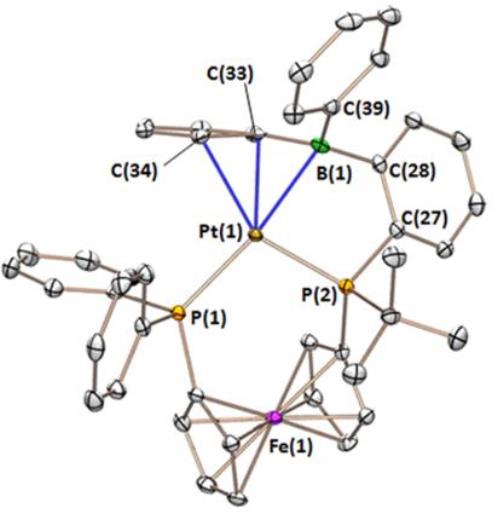
The FcPPB ligand η2BC-coordinated to platinum:
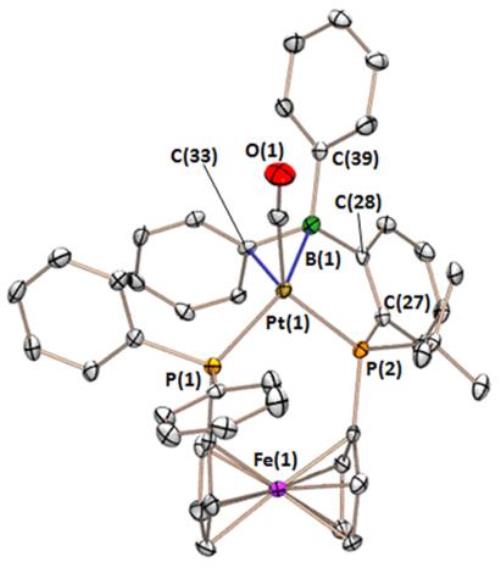
The FcPPB ligand η1B-coordinated to platinum:
|
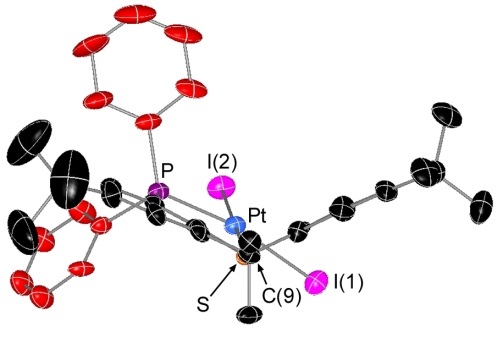
Rhodium Halide and PF6 Complexes of a Borane-Appended Ligand
The borane engages in a tight interaction to chloro or bromo co-ligands. By contrast, (a) there is only a weak interaction between the borane and iodo co-ligand, and (b) a fluoride co-ligand is completely abstracted to generate a zwitterionic complex in which the cationic rhodium center is now η2-coordinated to the ipso and ortho carbon atoms of one of the phenyl rings on boron:
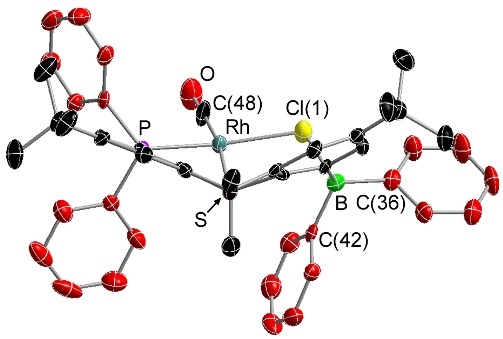
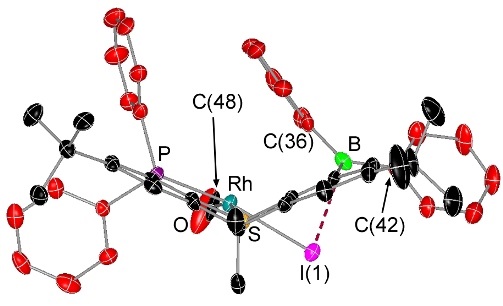
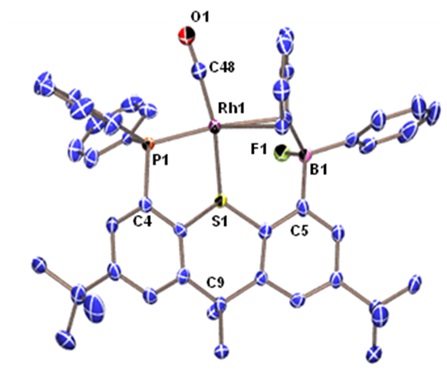
When the halide is entirely abstracted from the metal center, the borane engages in an η2BC-interaction with rhodium:
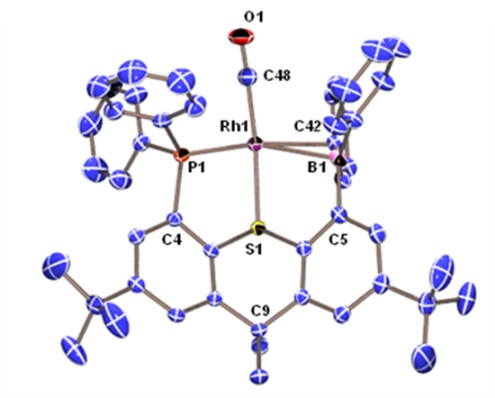
|
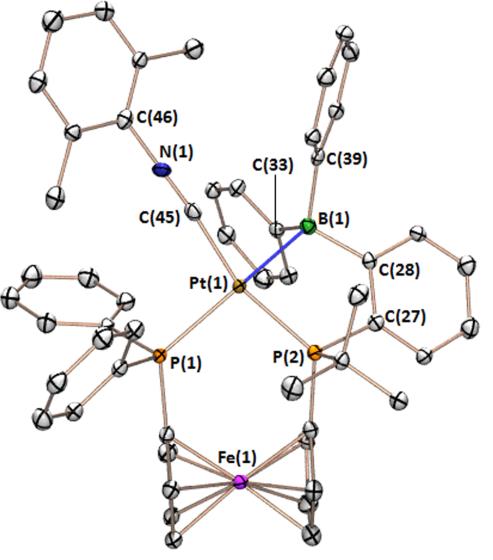
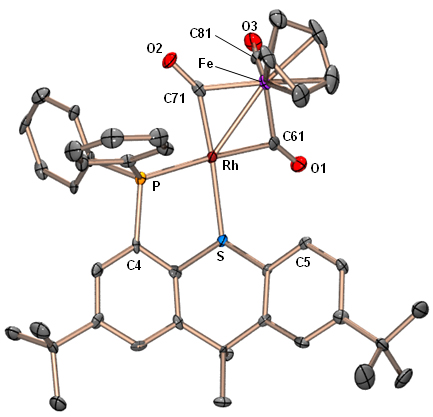
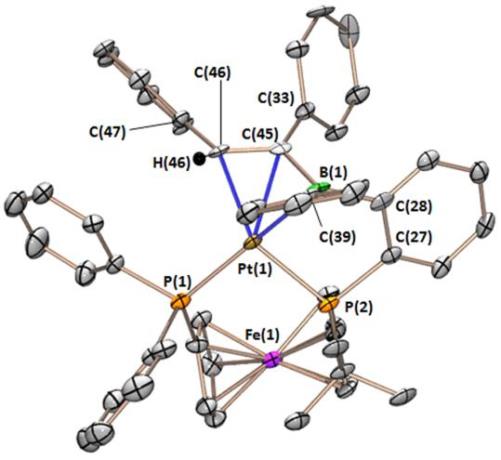
Rhodium-Iron Heterobimetallic Complexes of the TXPB Ligand
Reaction of [RhCl(CO)(TXPB)] with K[CpFe(CO)2] provided [(TXPB)Rh(μ-CO)2Fe(CO)Cp] in which the borane is η3BCC-coordinated to rhodium:
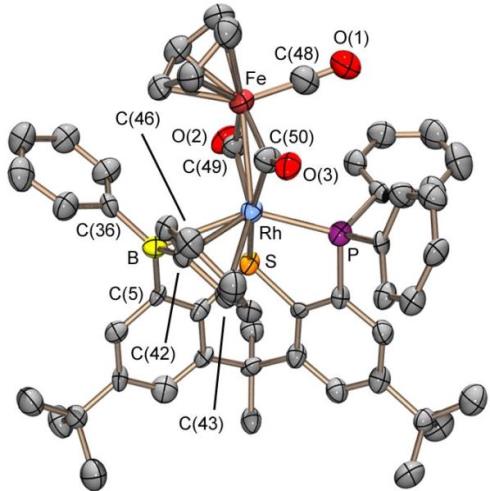
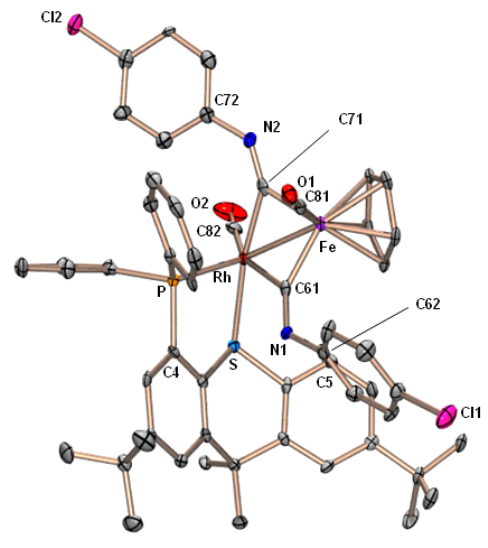
Reaction of [(TXPB)Rh(μ-CO)2Fe(CO)Cp] with isocyanides yields complexes featuring a bridging borataaminocarbyne (C–NR–BR3) ligand:
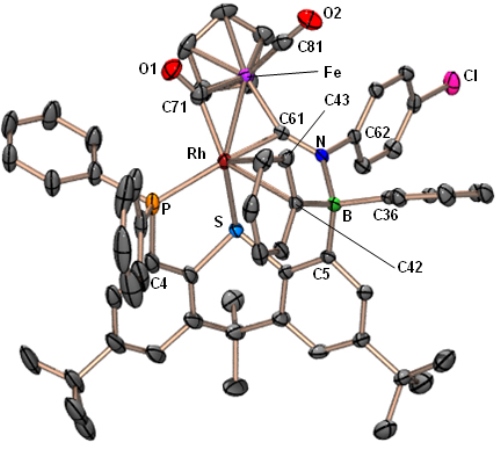
|
Palladium Complexes of a Phosphine-Thioether-Borane Ligand
The borane of the TXPB ligand is η3BCC-coordinated to palladium:
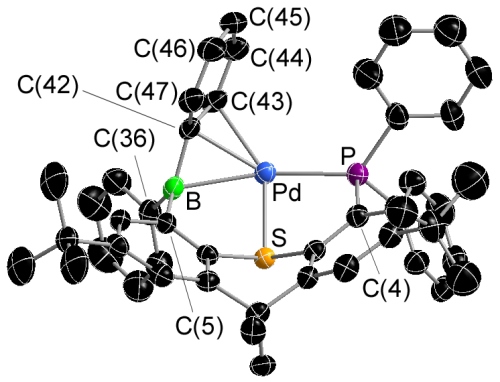
The TXPB ligand is κ1P-coordinated to palladium (the thioether and the borane are now uncoordinated):
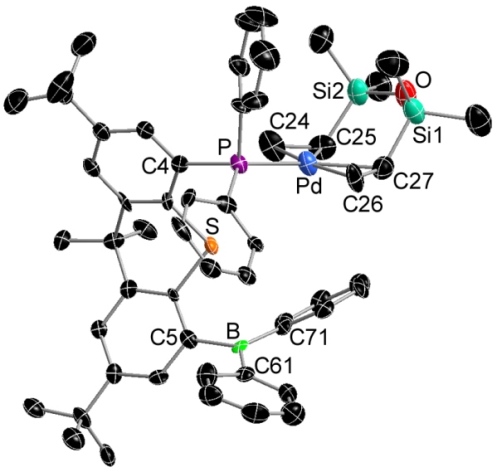
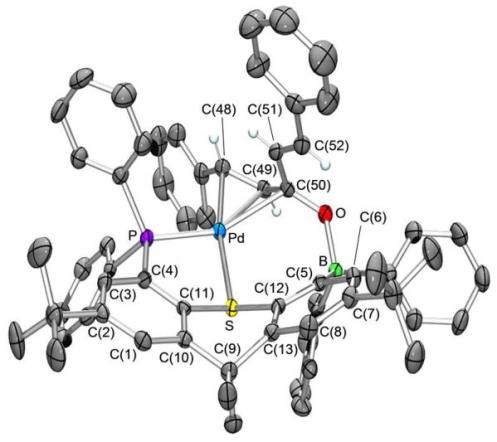
One molecule of dba (dibenzylideneacetone) is bound between palladium and the borane of the TXPB ligand. The resulting complex is best described as a zwitterionic boratoxyallyl complexes in which the allyl unit is η3-coordinated to palladium:
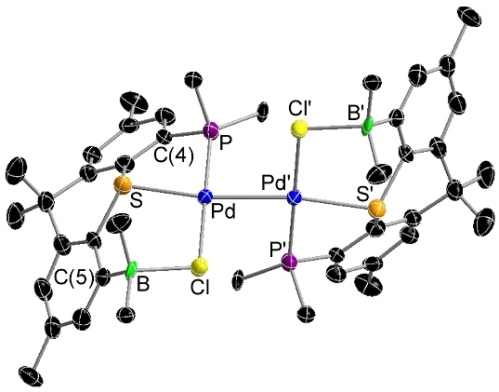
One-electron reduction of [PdCl2(TXPB)] yielded the bright red palladium dimer [{PdCl(TXPB)}2] shown below:
|
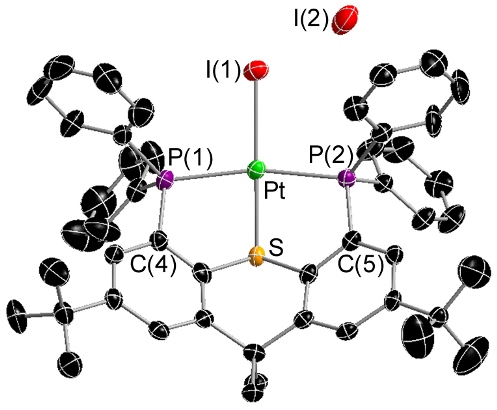
Alkyl and Aryl Complexes of Borane-Appended Ligands
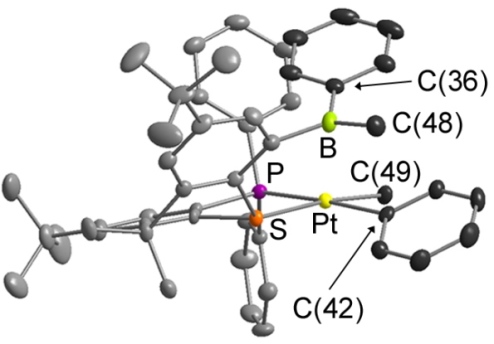
Reaction of TXPB with [PtMe2(COD)] provided the complex shown below, [PtMePh(TXPB')], with one phenyl and one methyl group on platinum, and one phenyl and one methyl group on boron.:
Heating [PtMePh(TXPB')] resulted in exchange of the remaining phenyl group on boron with the remaining methyl group on platinum:
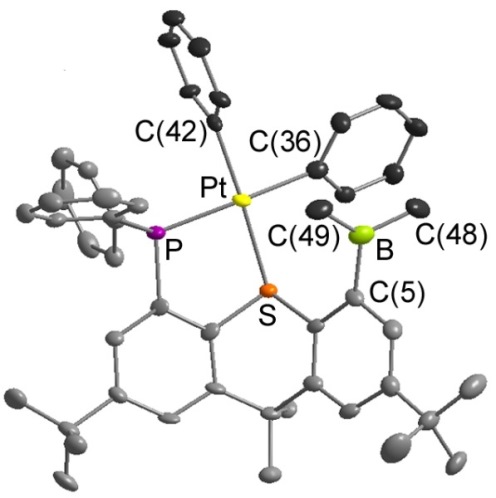
In solution, [PtMePh(TXPB')] exists in equilibrium with zwiterionic [PtMe(TXPB-Me)]. Neutral [PtMePh(TXPB')] is trapped by addition of 1 equivalent of P(OPh)3, while zwitterionic [PtMe(TXPB-Me)] is trapped by addition of 2 equivalents of CNXyl:
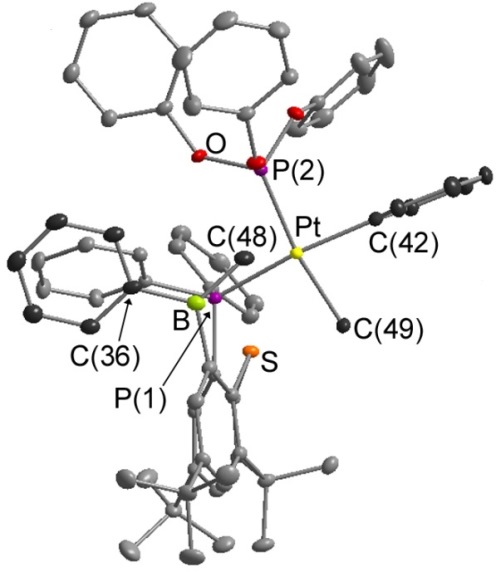
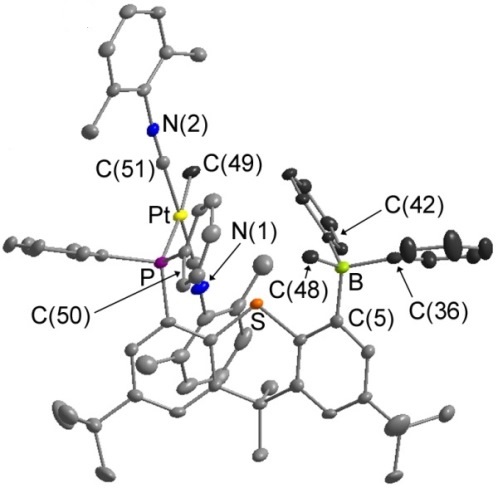
|
Complexes of PS- and PSP-Donor Ligands
The PS- and PSP-ligands in the complexes below are borane-free analogues of our borane-containing TXPB ligand. Our primary interest in these complexes is for comparison of their structures and reactivities with TXPB analogues.
|
Vinylborane Complexes
This is the first η3BCC-coordinated vinylborane complexes. The first one below binds like an allyl ligand, with extensive delocalization within the BCC core of the ligand. The second one below binds like an alkyl anion with an attached borataalkene group.
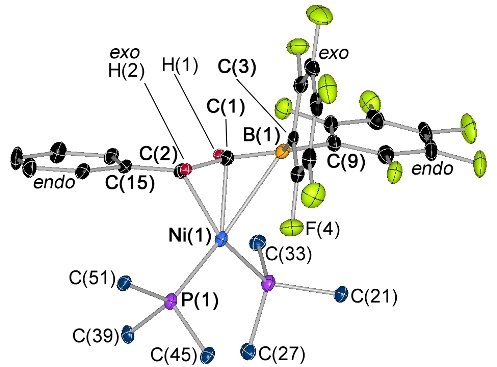
This is the first complex of a vinylborane-containing ambiphilic ligand (it was formed in situ via the reaction of [Pt(FcPPB)] with HC2Ph):
|
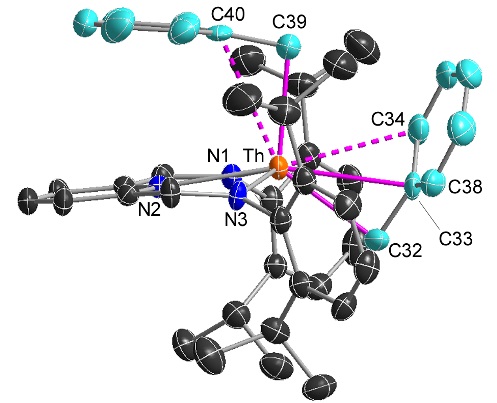
Rigid Ligand Thorium Complexes
Neutral thorium dialkyl complexes (in the dibenzyl complex, the hydrocarbyl ligands are η2- or η3-coordinated):
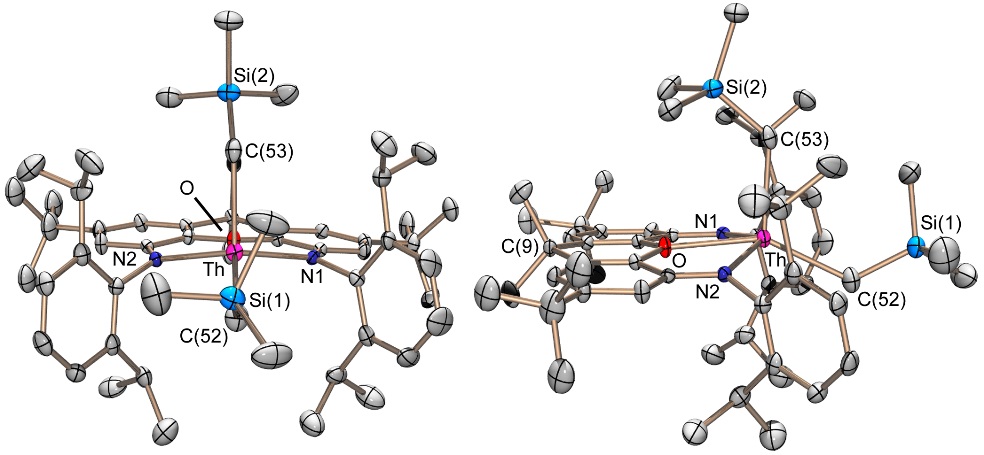
An unexpected adduct between [(BDPP)ThX2] and MeMgX(OEt2) where X = Cl or Br, and BDPP = an NNN-donor pincer ligand):
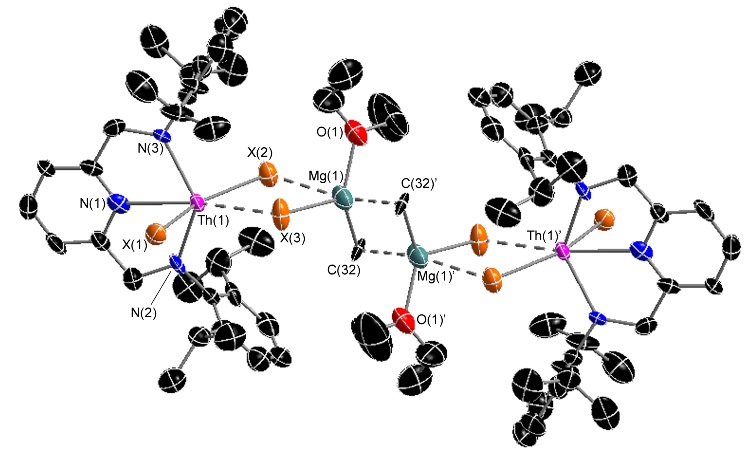
A rare example of a thorium alkyl cation (the 2nd view shows packing between the cations and B(C6F5)4 anions):
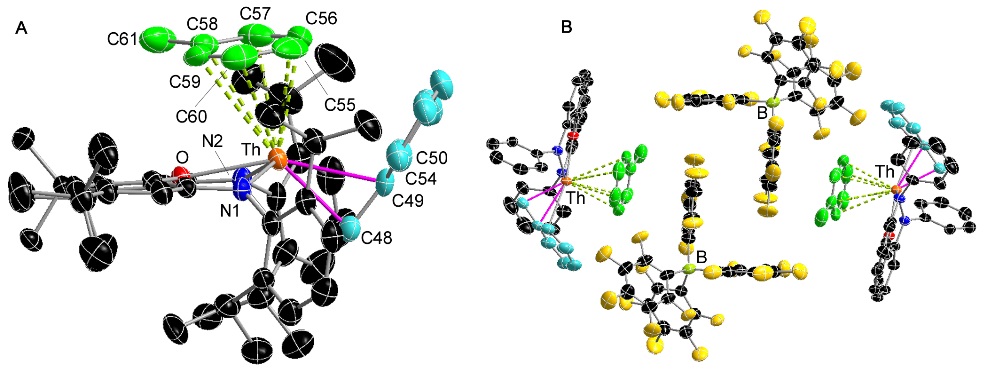
A cationic thorium alkyl complex coordinated to a molecule of the neutral [(XA2)Th(CH2Ph)2] starting material. The μ-η1:η6-coordination mode in this structure is a previously unknown coordination mode for a benzyl ligand:
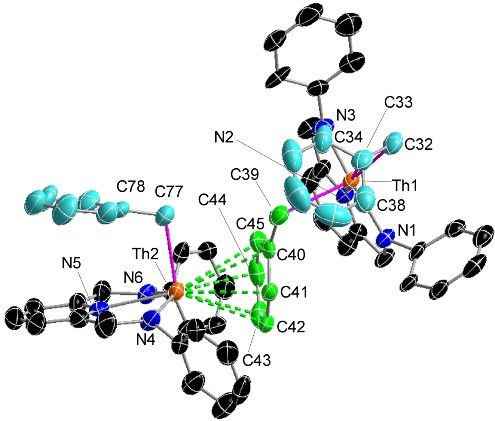
A thorium dication formed by double alkyl abstraction. The dictionic metal center is π-coordinated to two PhCH2B(C6F5)3 anions.
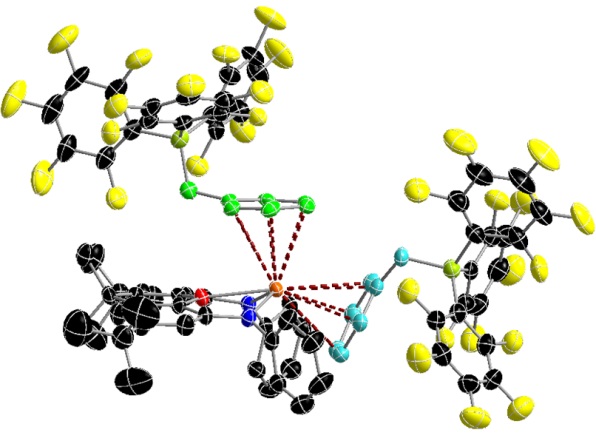
A very sterically hindered complex featuring two BDPP pincer ligand dianions coordinated to thorium(IV):
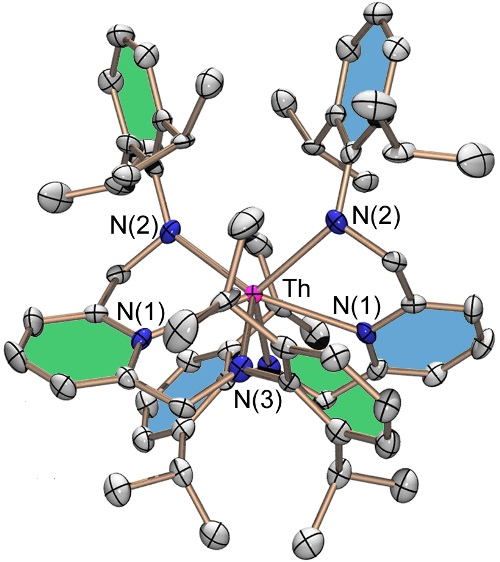
|
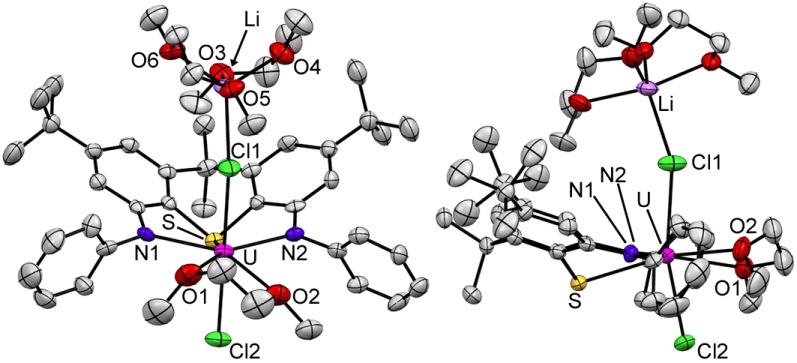
Rigid Ligand Uranium Complexes
Uranium(IV) halide complexes of rigid NON- and NSN-donor ligands (2 views of each):
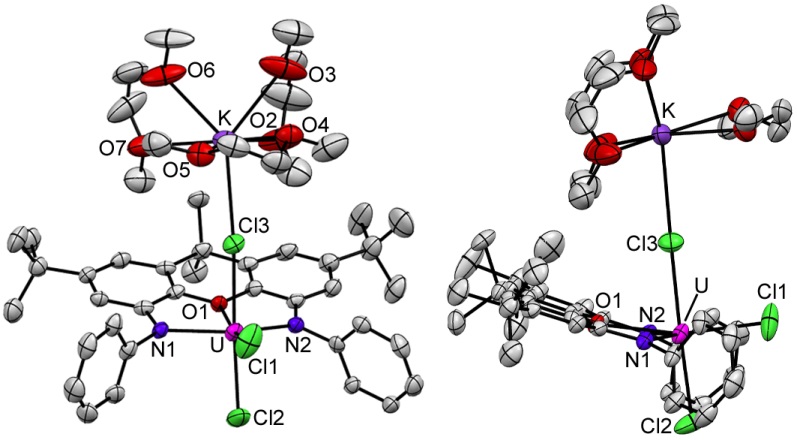
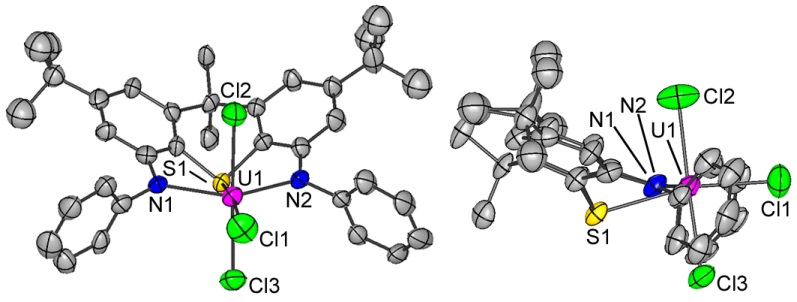
Uranium(III) halide complexes of rigid NON- and NSN-donor ligands (2 views of each):
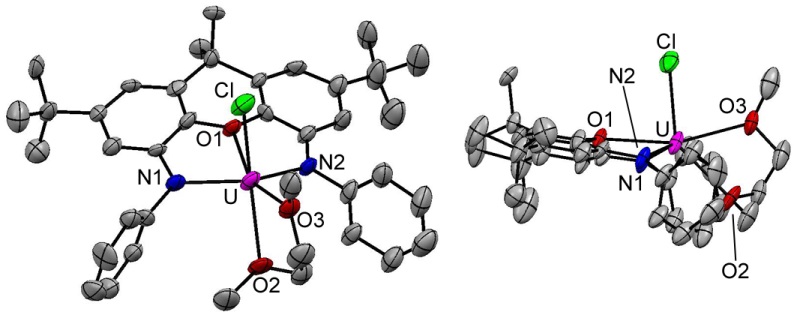
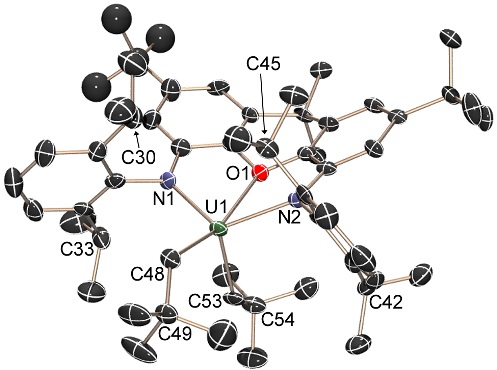
A representative uranium(IV) alkyl complex of our most commonly used NON-donor pincer ligand, XA2:
|
Yttrium Complexes
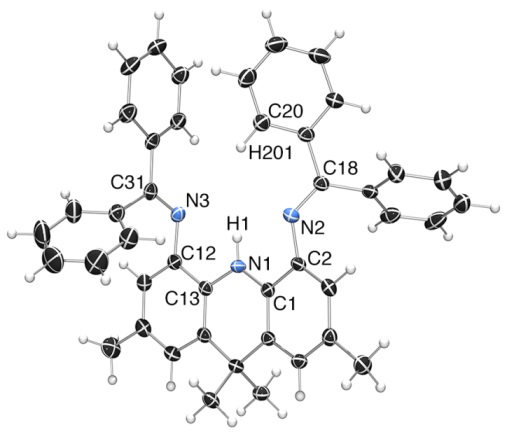
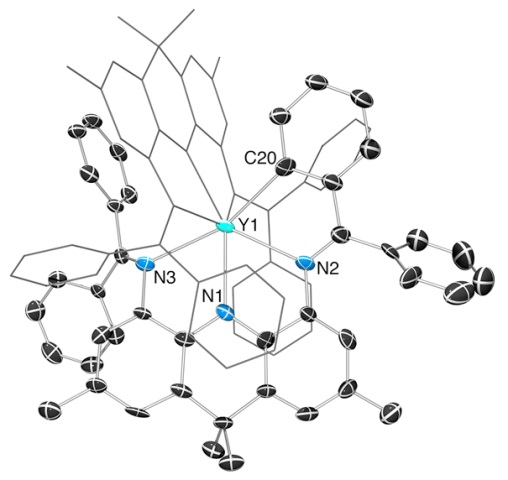
We prepared the first acridanide-backbone pincer ligand (the AIm2 monoanion). However, it was readily cyclometallated in the coordination sphere of yttrium, and then two ligands (the cyclometallated ligand and the intact ligand) are coupled together... The low thermal stability of the complexes stems from the imine donors, not the acridanide backbone...
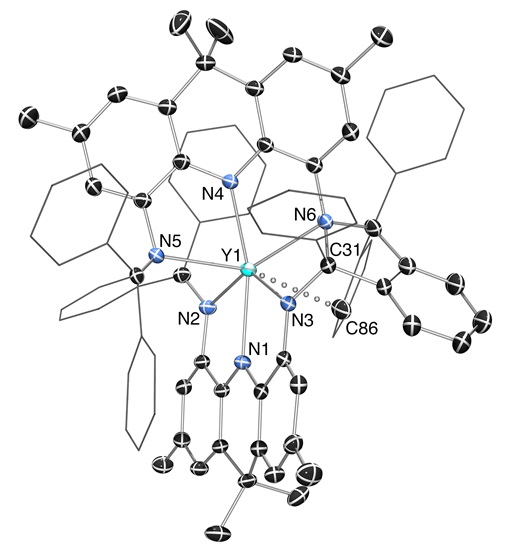
|
Interesting Alkali Metal Complexes
Potassium-alkane interactions!!! (these are the first crystallographically characterized main group-alkane interactions):
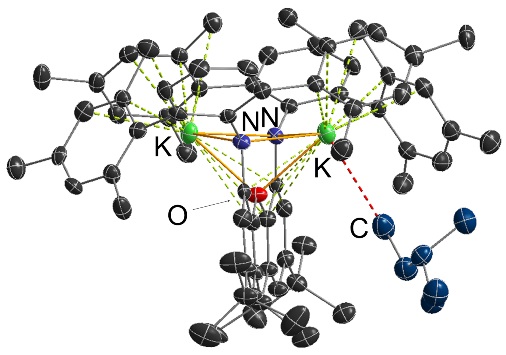
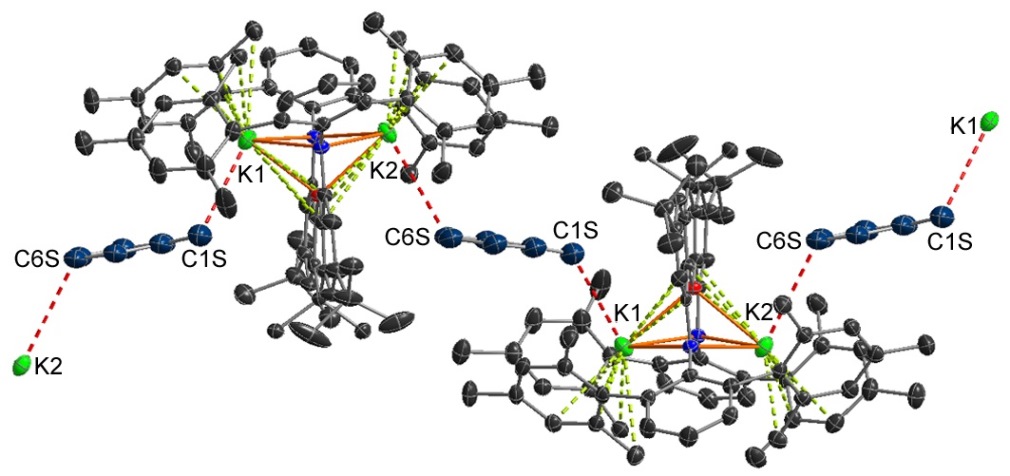
An unexpected adduct between nBuLi and Li2[XAT]:
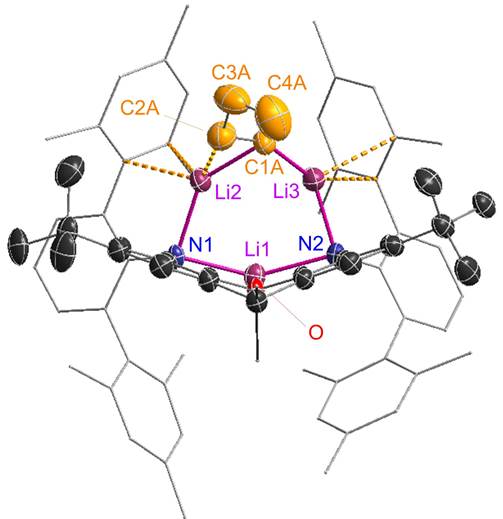
|
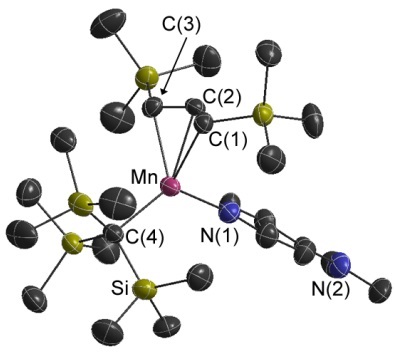
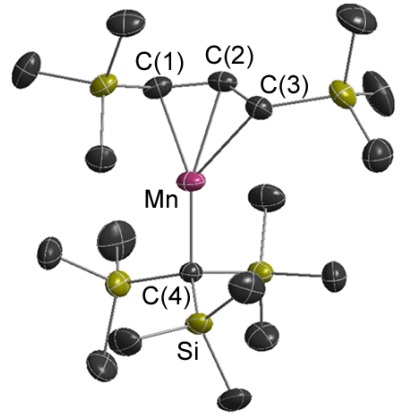
Manganese Alkyl/Allyl Complexes
Volatile and thermally robust mixed alkyl / allyl complexes. Their thermal stability is remarkable given their high spin d5 configuration!
|
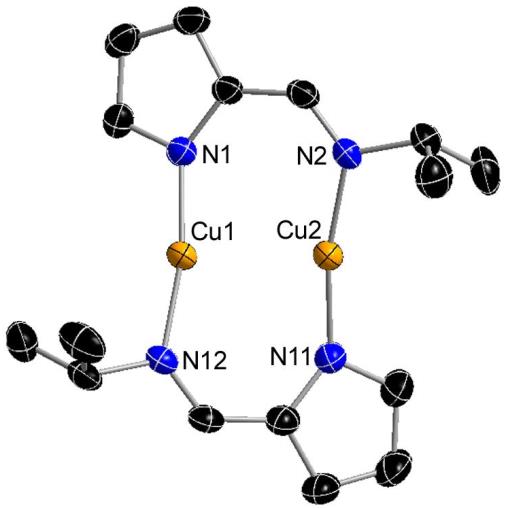
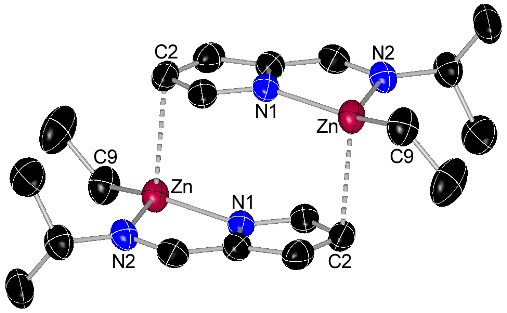
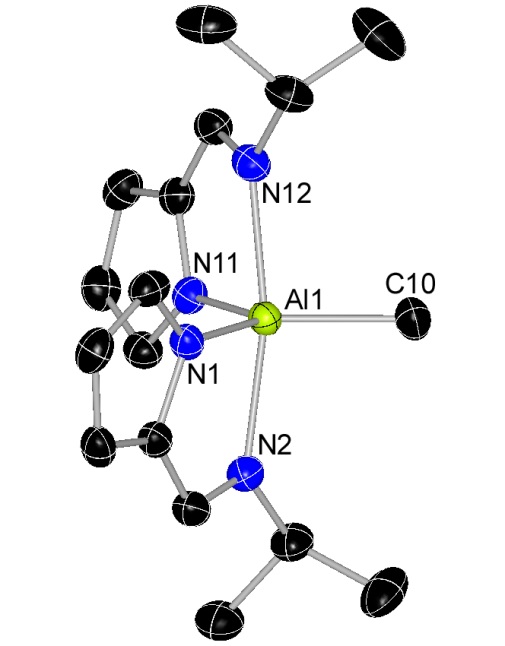
Dimetallic Copper and Zinc Complexes
The complexes below are formed as intermediates in the reactions of [CuL2] (L = N-isopropyl-2-pyrrolylaldimine) with ZnEt2 or AlMe3 to deposit copper metal.
|





















































